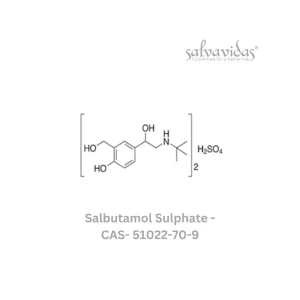
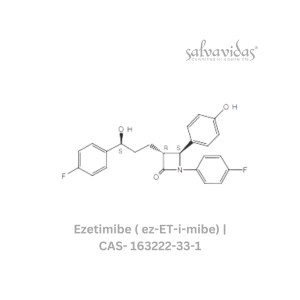
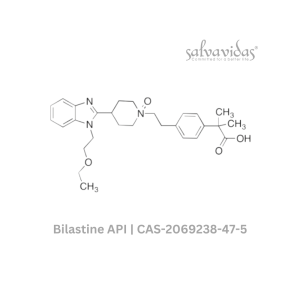
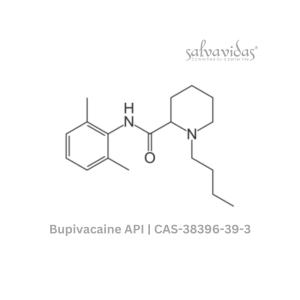
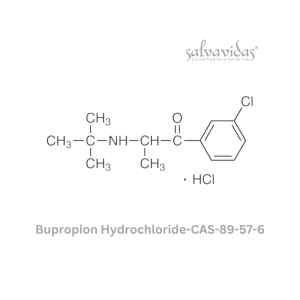
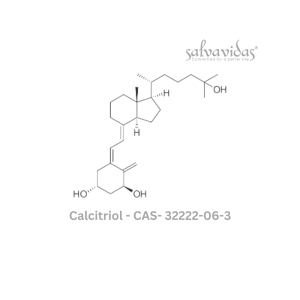
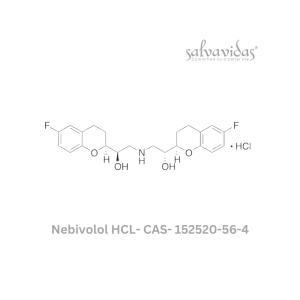
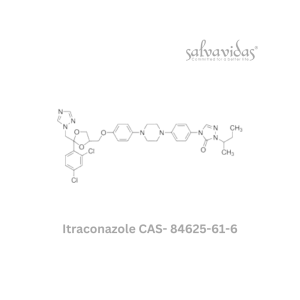
49
05
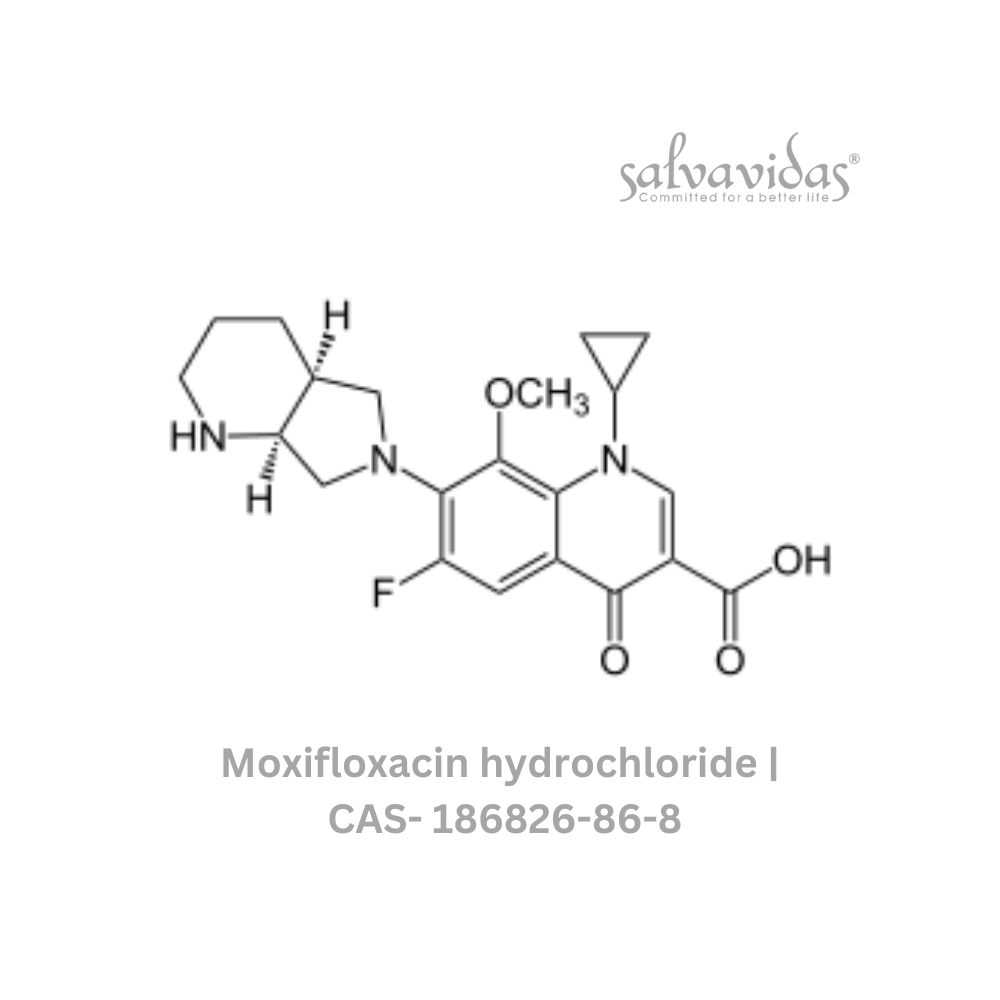
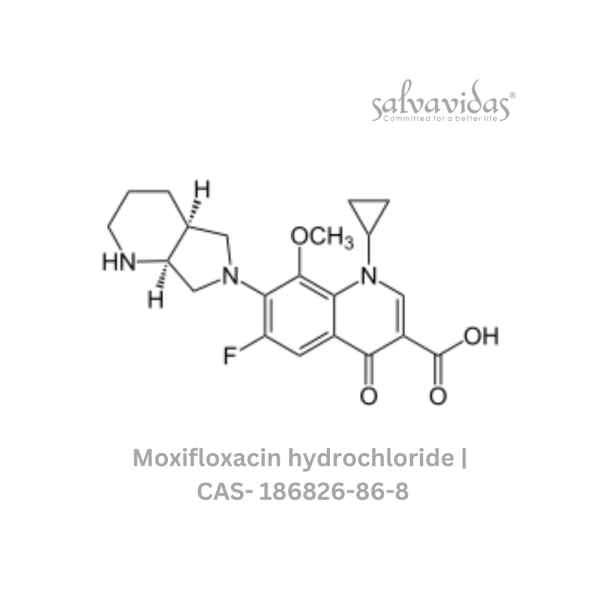
Moxifloxacin hydrochloride
“The U.S. Food and Drug Administration (FDA) has approved the antibacterial prescription drug moxifloxacin hydrochloride for the treatment of specific bacterial infections, including community-acquired pneumonia, acute worsening of chronic bronchitis, acute sinus infections, plague, and skin and abdominal infections.
Moxifloxacin Tablets should be taken 400 mg (orally) once every 24 hours. Depending on the infection kind, the course of treatment may vary.
In patients receiving therapy with fluoroquinolones, including Moxifloxacin Tablets, significant and occasionally fatal adverse events, some related to hypersensitivity and some due to unclear etiology, have been recorded.
The use of moxifloxacin tablets may result in side effects that are fatal (such as hives, difficulty breathing or swallowing, swelling of the lips, tongue, or face), as well as less serious adverse effects.
Use of Moxifloxacin Tablets for conditions for which they are not prescribed is prohibited. Even if they have the same symptoms as you do, avoid giving other individuals Moxifloxacin Tablets. It might hurt them.
*Products will not be offered for sale in countries where valid Patents are in force.
* It is the responsibility of the buyer to comply with the above.
| Generic Name : | Moxifloxacin hydrochloride |
|---|---|
| CAS Number : | 186826-86-8 |
| Grade : | IP/BP/USP |
| Packing Type : | Drum |
| Therapeutic use : | Antibactrial agents |
| Product MOQ : | 25 kg |
Product Inquiry Form
Your details will not be published