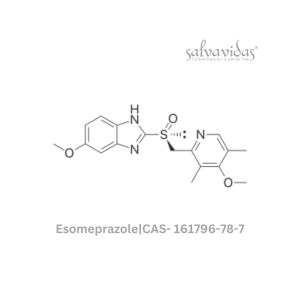
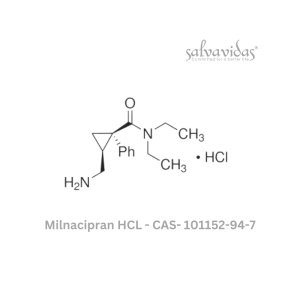
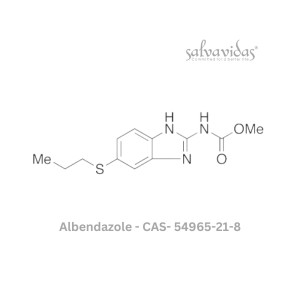
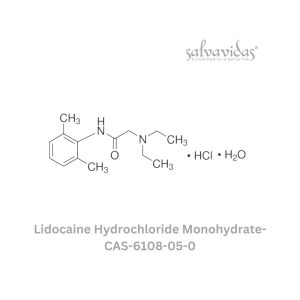
49
05
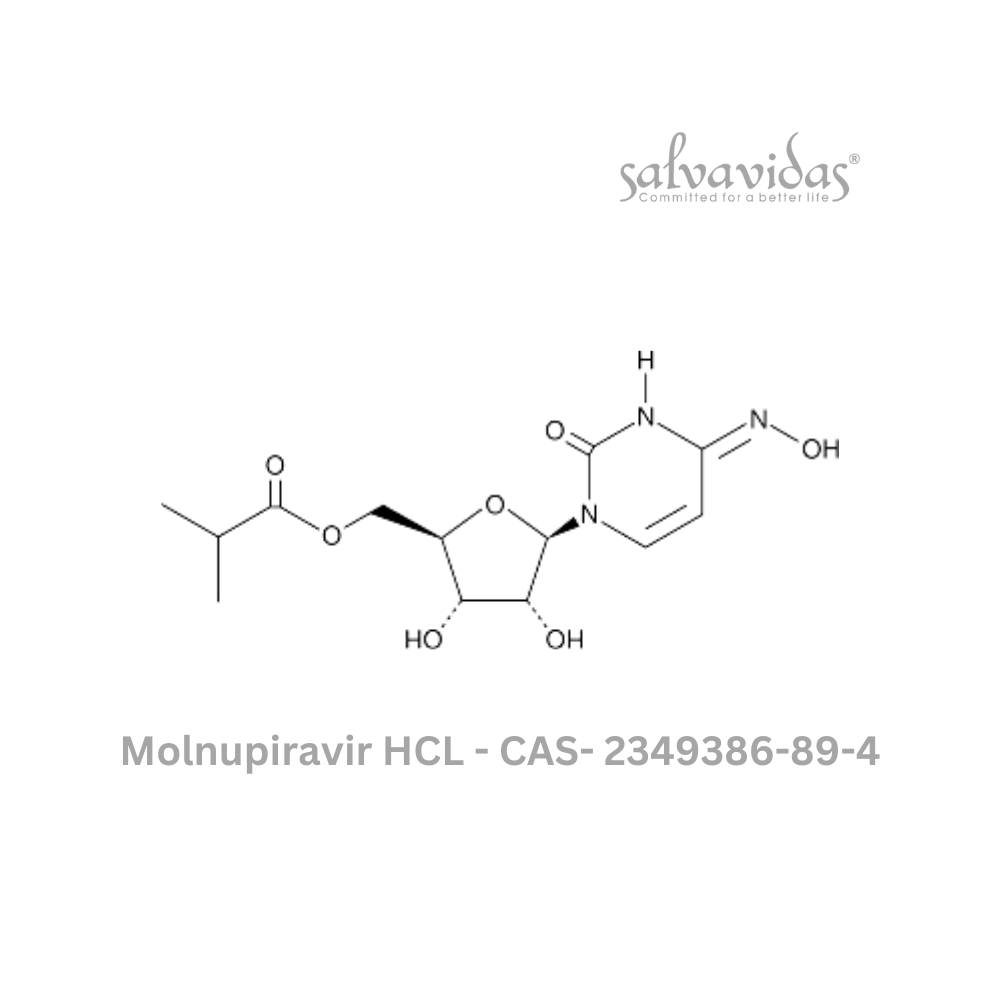
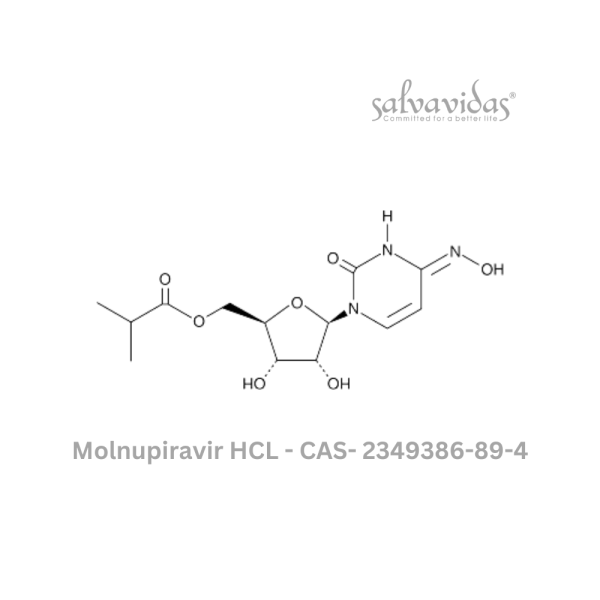
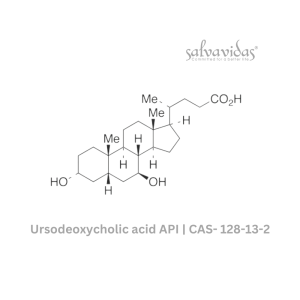
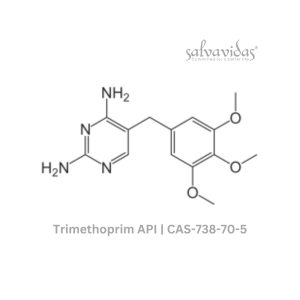
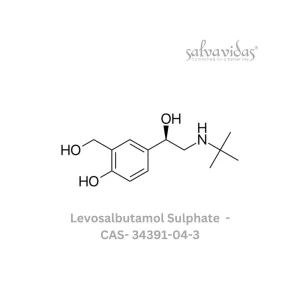
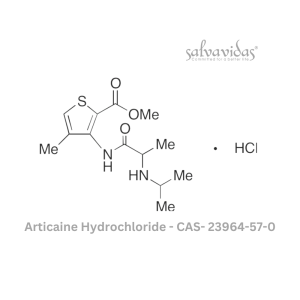
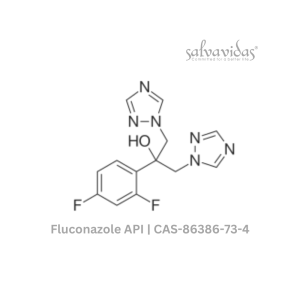
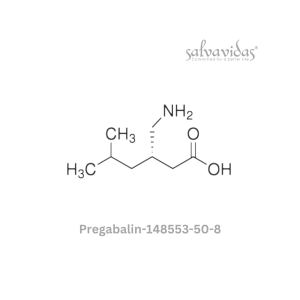
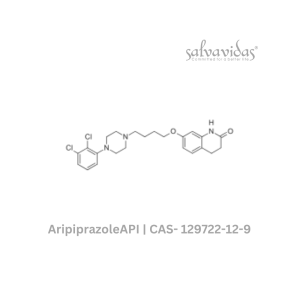
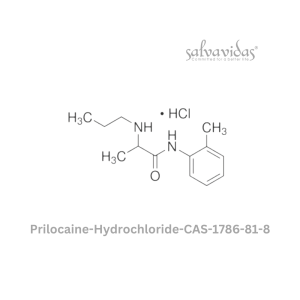
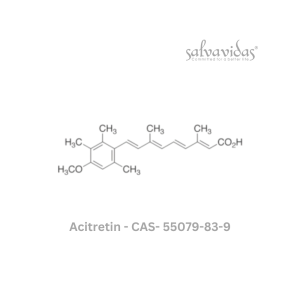
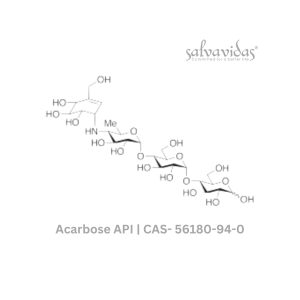
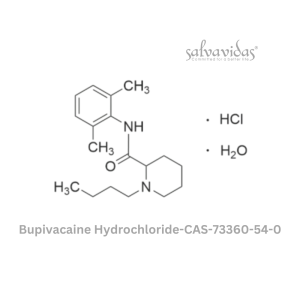
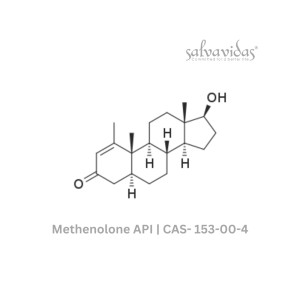
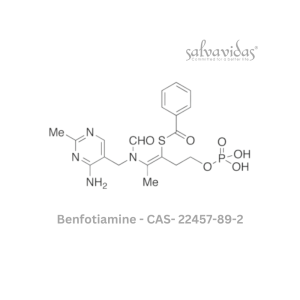
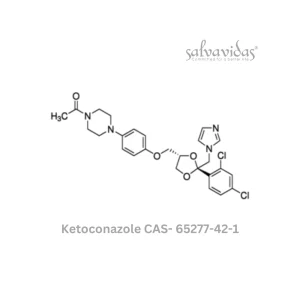
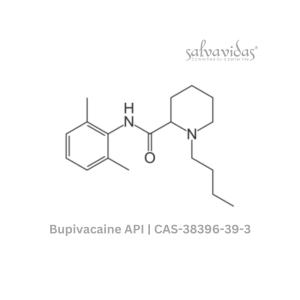
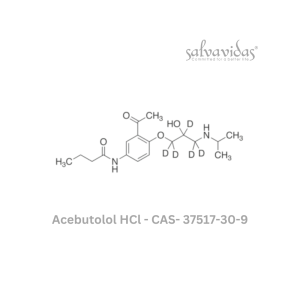
Molnupiravir API
An investigational drug called molnupiravir is being researched for the treatment of mild-to-moderate COVID-19. There are still hazards associated with this medication that are being investigated.
Molnupiravir has been approved for use in the treatment of mild-to-moderate COVID-19 in adults 18 years of age and older by the US Food and Drug Administration (FDA).
The use of molnupiravir will not reduce your risk of spreading infectious diseases to others. Continue utilizing infection prevention techniques including keeping to yourself, avoiding close contact with people, cleaning your hands, wearing a face mask for protection, sanitizing frequently touched surfaces, and not sharing personal belongings.
*Products will not be offered for sale in countries where valid Patents are in force.
* It is the responsibility of the buyer to comply with the above.
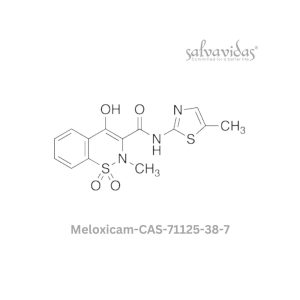
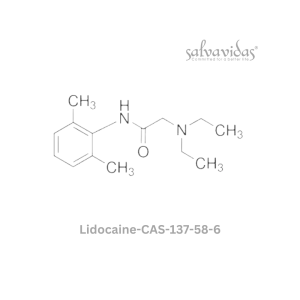
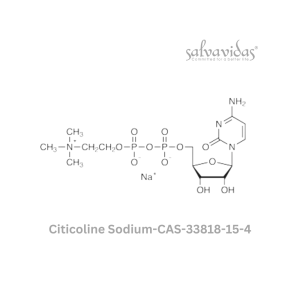
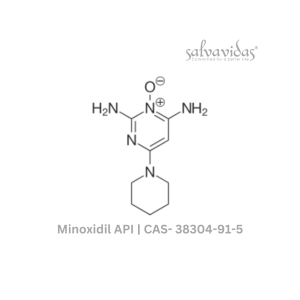
| Generic Name : | Molnupiravir API |
|---|---|
| CAS Number : | 2349386-89-4 |
| Grade : | IP / BP / USP |
| Packing Type : | Drum |
| Therapeutic use : | Miscellaneous antivirals |
| Product MOQ : | 1-kg |
Product Inquiry Form
Your details will not be published